

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Squalene synthase (Rattus norvegicus) | BDBM50029174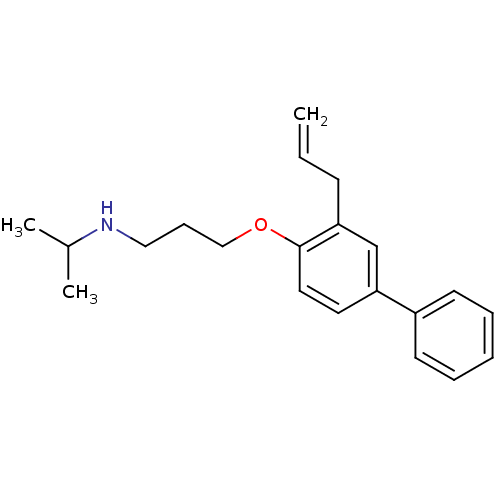 (CHEMBL131973 | N-(1-methylethyl)-3-[(3-prop-2-en-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PubMed | n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038093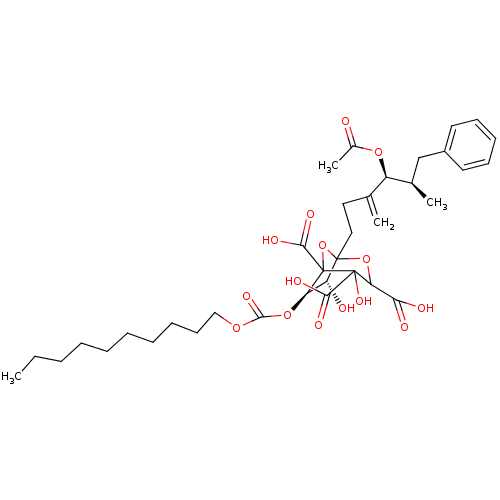 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038102 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029177 (CHEMBL134337 | Propionic acid 3-allyl-4-(3-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038087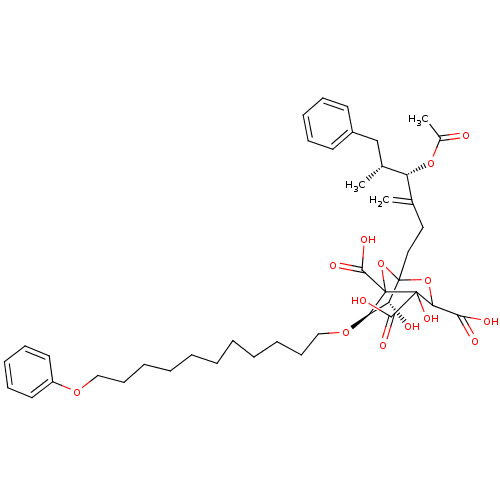 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038138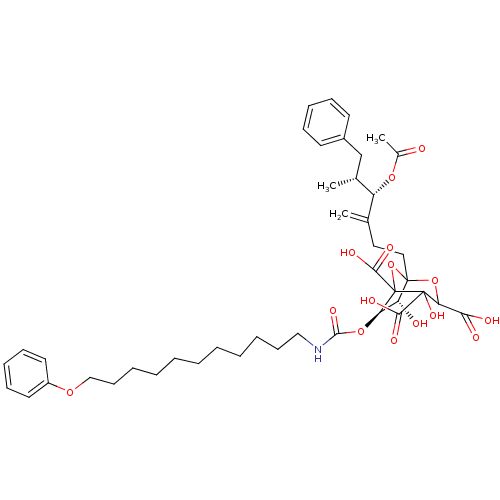 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038092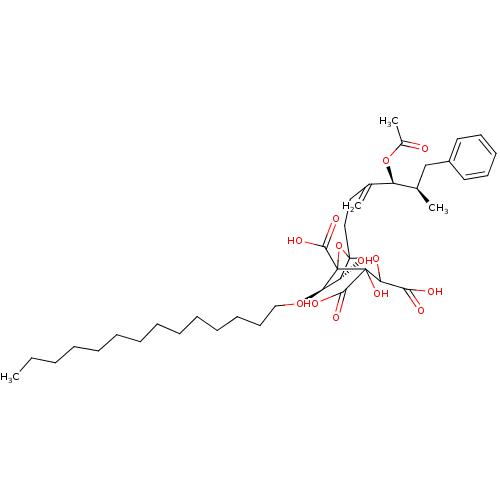 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038089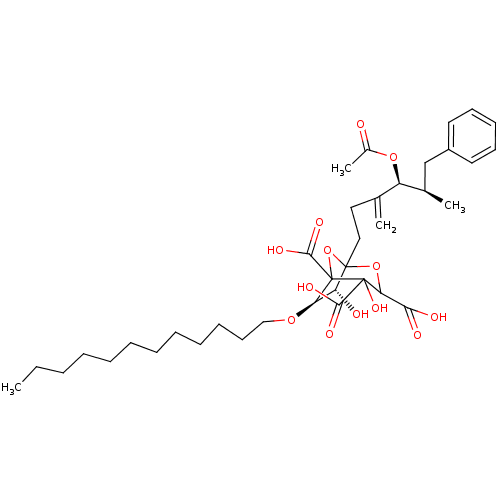 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038120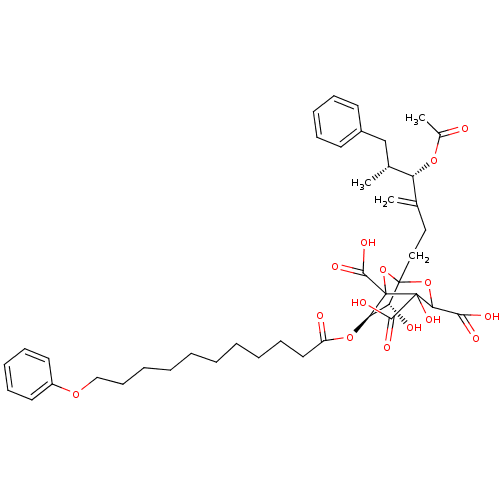 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038123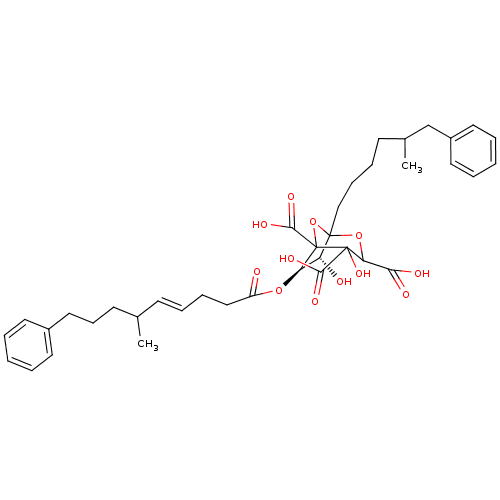 ((6R,7R)-4,7-Dihydroxy-1-(5-methyl-6-phenyl-hexyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038122 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038121 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038091 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038151 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038125 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038090 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50292336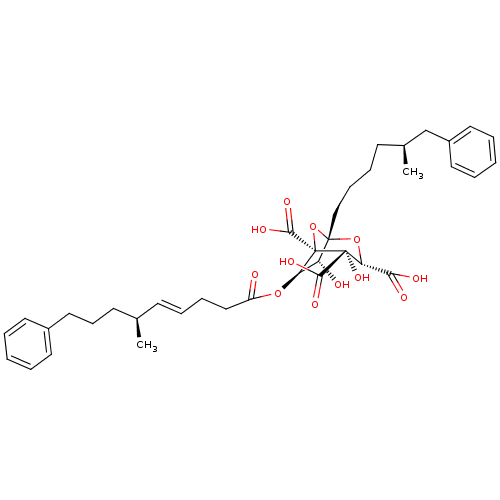 (CHEMBL507677 | desacetoxy-zaragozic acid) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of squalene synthase | J Nat Prod 59: 52-54 (1996) Article DOI: 10.1021/np960003i BindingDB Entry DOI: 10.7270/Q2Q52PND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50292415 (CHEMBL504845 | Zaragozic Acid B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of rat liver squalene synthase by liqiud scintillation counting | J Nat Prod 56: 1923-1929 (1993) Article DOI: 10.1021/np50101a009 BindingDB Entry DOI: 10.7270/Q2M908Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029166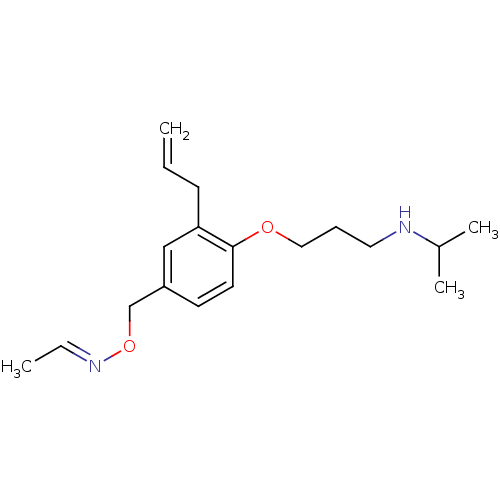 (Acetaldehyde O-[3-allyl-4-(3-isopropylamino-propox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038119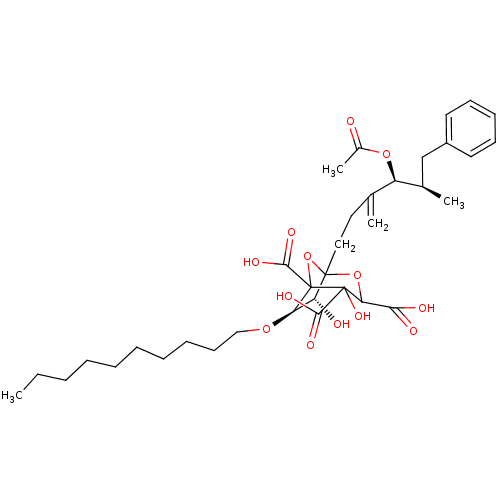 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038096 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50038096 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HepG2 Squalene Synthase (SQS) | Bioorg Med Chem Lett 5: 1989-1994 (1995) Article DOI: 10.1016/0960-894X(95)00339-U BindingDB Entry DOI: 10.7270/Q2B56K6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038103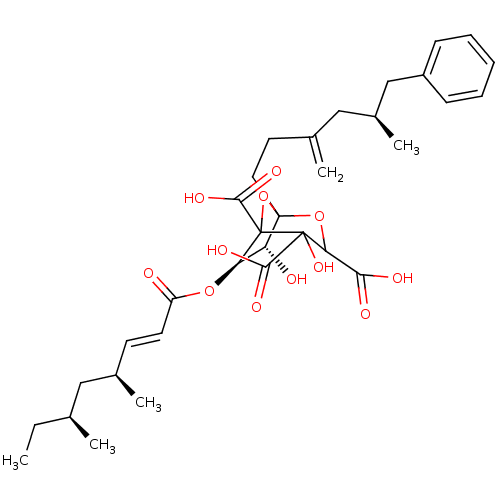 ((6R,7R)-6-((E)-(4S,6S)-4,6-Dimethyl-oct-2-enoyloxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038114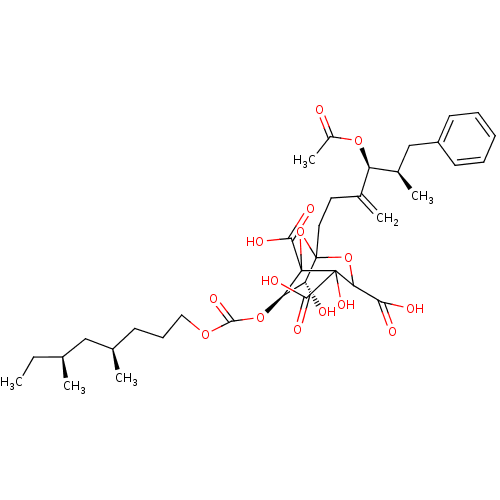 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50038102 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description compound required to inhibit 50% activity of yeast squalene synthase enzyme (YESS) | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038135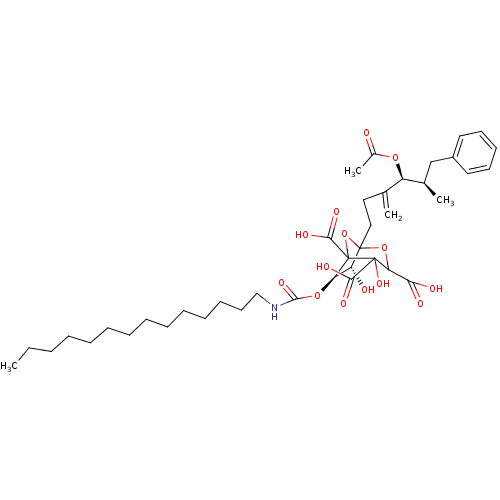 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038096 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat squalene synthase | Bioorg Med Chem Lett 5: 2403-2408 (1995) Article DOI: 10.1016/0960-894X(95)00419-T BindingDB Entry DOI: 10.7270/Q2NK3F0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50038089 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description compound required to inhibit 50% activity of yeast squalene synthase enzyme (YESS) | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50292333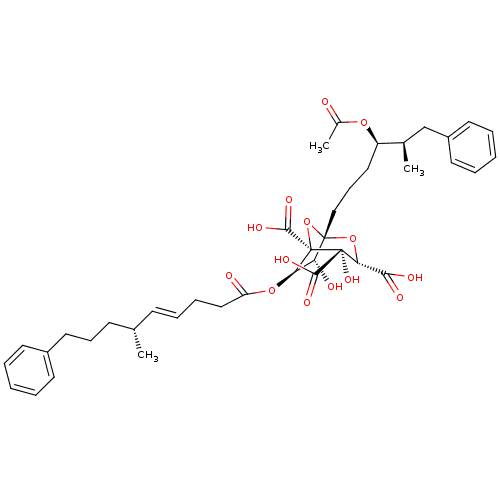 (CHEMBL505374 | zaragozic acid C) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of squalene synthase | J Nat Prod 59: 52-54 (1996) Article DOI: 10.1021/np960003i BindingDB Entry DOI: 10.7270/Q2Q52PND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50442114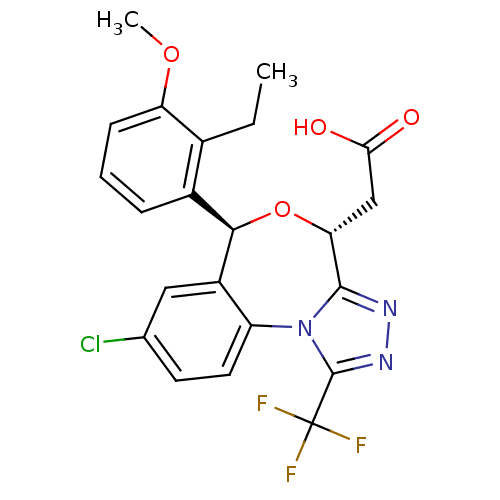 (CHEMBL2441090) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometry | ACS Med Chem Lett 4: 932-6 (2013) Article DOI: 10.1021/ml400151c BindingDB Entry DOI: 10.7270/Q2J67JCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038127 ((6R,7R)-1-(4-Acetoxy-5-methyl-6-phenyl-hexyl)-4,7-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038128 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50292333 (CHEMBL505374 | zaragozic acid C) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of rat liver squalene synthase by liqiud scintillation counting | J Nat Prod 56: 1923-1929 (1993) Article DOI: 10.1021/np50101a009 BindingDB Entry DOI: 10.7270/Q2M908Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038104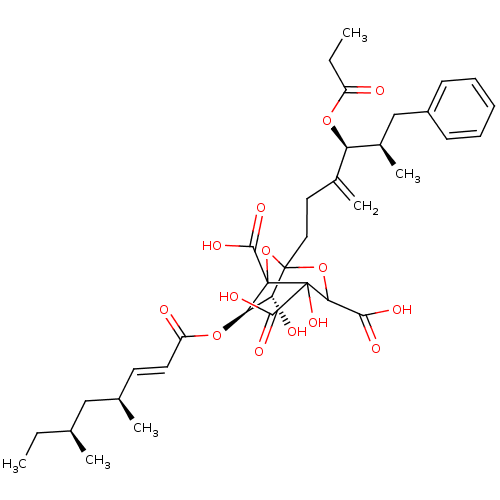 ((6R,7R)-6-((E)-(4S,6S)-4,6-Dimethyl-oct-2-enoyloxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50338903 (CHEMBL1684849 | Ethyl 1-{4-[{4-chloro-2-[(2-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of squalene synthase | Bioorg Med Chem 19: 1930-49 (2011) Article DOI: 10.1016/j.bmc.2011.01.065 BindingDB Entry DOI: 10.7270/Q2ZG6SJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50051873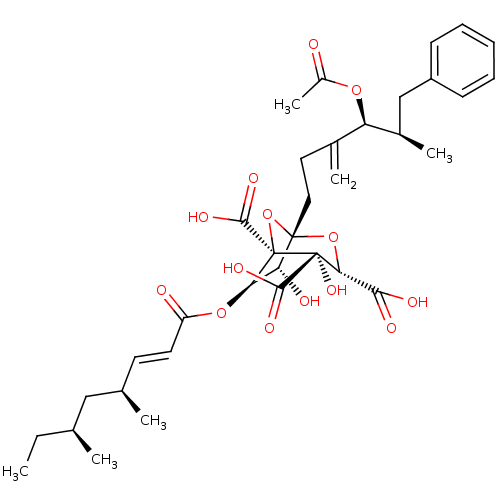 ((1S,3S,4S,5R,6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of rat liver squalene synthase by liqiud scintillation counting | J Nat Prod 56: 1923-1929 (1993) Article DOI: 10.1021/np50101a009 BindingDB Entry DOI: 10.7270/Q2M908Q1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50388376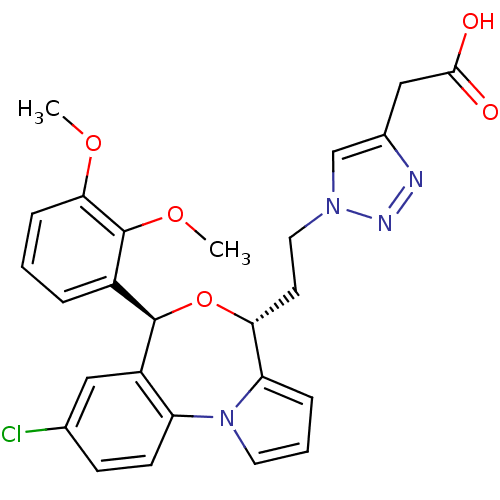 (CHEMBL2057637) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of squalene synthase in rat hepatic cells | Bioorg Med Chem 20: 3072-93 (2012) Article DOI: 10.1016/j.bmc.2012.02.054 BindingDB Entry DOI: 10.7270/Q2B8595B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038101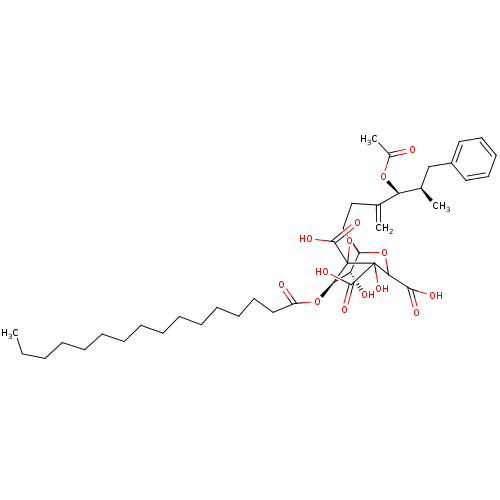 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50285068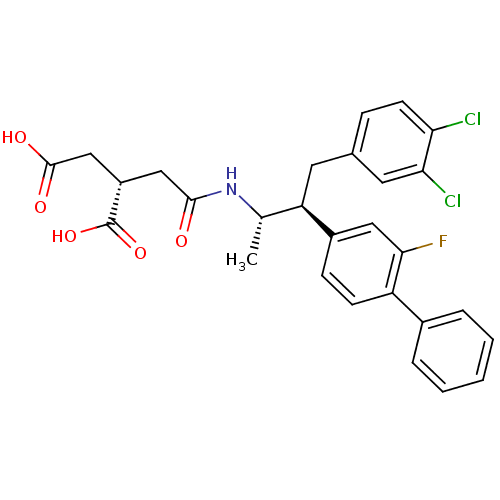 ((S)-2-{[(1S,2S)-3-(3,4-Dichloro-phenyl)-2-(2-fluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against squalene synthase (SQS) obtained from HepG2 cells | Bioorg Med Chem Lett 6: 463-466 (1996) Article DOI: 10.1016/0960-894X(96)00033-9 BindingDB Entry DOI: 10.7270/Q2MG7PG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50285068 ((S)-2-{[(1S,2S)-3-(3,4-Dichloro-phenyl)-2-(2-fluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HepG2 Squalene Synthase (SQS) | Bioorg Med Chem Lett 5: 1989-1994 (1995) Article DOI: 10.1016/0960-894X(95)00339-U BindingDB Entry DOI: 10.7270/Q2B56K6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50388375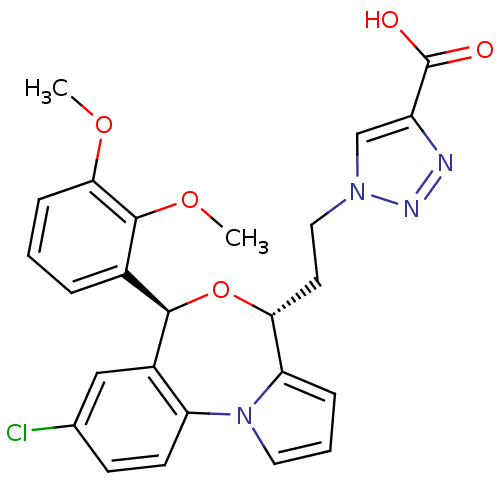 (CHEMBL2057636) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of squalene synthase in rat hepatic cells | Bioorg Med Chem 20: 3072-93 (2012) Article DOI: 10.1016/j.bmc.2012.02.054 BindingDB Entry DOI: 10.7270/Q2B8595B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029171 (CHEMBL341371 | N-[3-Benzyl-4-(3-isopropylamino-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50029159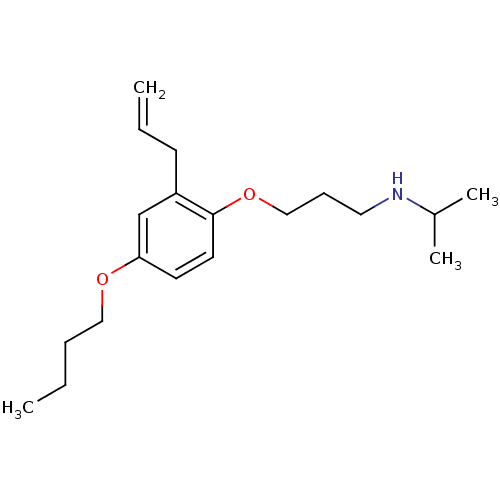 (CHEMBL132881 | [3-(2-Allyl-4-butoxy-phenoxy)-propy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of rat microsomal squalene synthase | J Med Chem 38: 4157-60 (1995) BindingDB Entry DOI: 10.7270/Q21V5D0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50038093 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description compound required to inhibit 50% activity of yeast squalene synthase enzyme (YESS) | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038088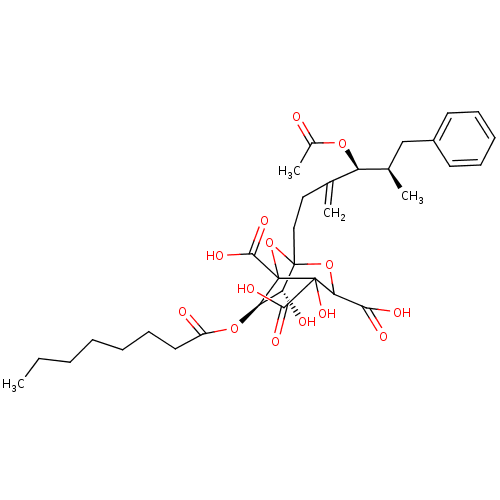 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition against squalene synthase enzyme in rat liver | J Med Chem 37: 4031-51 (1994) BindingDB Entry DOI: 10.7270/Q2PC31F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50388378 (CHEMBL2057639) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of squalene synthase in rat hepatic cells | Bioorg Med Chem 20: 3072-93 (2012) Article DOI: 10.1016/j.bmc.2012.02.054 BindingDB Entry DOI: 10.7270/Q2B8595B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50388348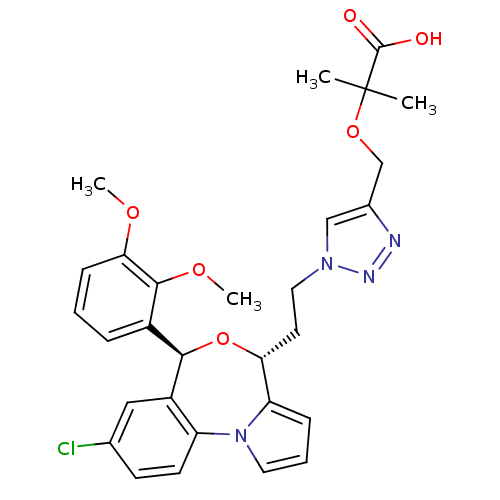 (CHEMBL2057952) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of squalene synthase in rat hepatic cells | Bioorg Med Chem 20: 3072-93 (2012) Article DOI: 10.1016/j.bmc.2012.02.054 BindingDB Entry DOI: 10.7270/Q2B8595B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031845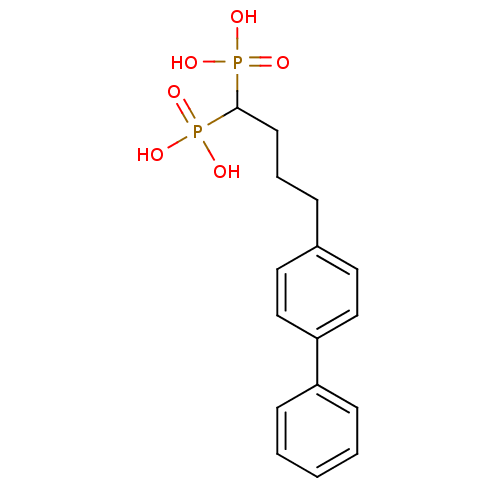 ((4-Biphenyl-4-yl-1-phosphono-butyl)-phosphonic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031845 ((4-Biphenyl-4-yl-1-phosphono-butyl)-phosphonic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50442115 (CHEMBL2440128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of rat hepatic microsomal squalene synthase using [3H]FPP as substrate after by scintillation spectrophotometry | ACS Med Chem Lett 4: 932-6 (2013) Article DOI: 10.1021/ml400151c BindingDB Entry DOI: 10.7270/Q2J67JCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 863 total ) | Next | Last >> |